- 1Stem Cells and Regenerative Medicine Lab, Fondazione Istituto di Ricerca Pediatrica Città Della Speranza, Padova, Italy
- 2Department of Women and Children Health, University of Padova, Padova, Italy
- 3First Surgical Clinic, Department of Surgery, Oncology and Gastroenterology, University of Padova, Padova, Italy
- 4NanoInspired Biomedicine Lab, Fondazione Istituto di Ricerca Pediatrica Città della Speranza, Padova, Italy
- 5LIFELAB Program, Consorzio per la Ricerca Sanitaria-CORIS, Padova, Italy
Cells and extracellular matrix (ECM) components represent the multifaceted and dynamic environment that distinguishes each organ. Cancer is characterized by the dysregulation of the composition and structure of the tissues, giving rise to the tumor milieu. In this review, we focus on the microenvironmental analysis of colorectal cancer (CRC) and rhabdomyosarcoma (RMS), two different solid tumors. While a lot is known about CRC environment, for RMS, this aspect is mostly unexplored. Following the example of the more complete CRC microenvironmental characterization, we collected and organized data on RMS for a better awareness of how tissue remodeling affects disease progression.
Introduction
For many years, the cellular compartment has been considered the main target both to classify the disease and to develop a therapy. However, in the past two decades, the role of the non-cellular part, the extracellular matrix (ECM), has been taken into consideration as a key element that, together with cells, contributes in TME building (1).
Fibroblasts are the main players in ECM remodeling. In physiological conditions, they remain quiescent, but in wound healing response, they can transiently activate to myofibroblasts and participate in synthesis and deposition of ECM proteins (2). The activated fibroblasts in the tumor microenvironment have different features compared to myofibroblasts and are called cancer-associated fibroblasts (CAFs). CAFs in tumor stroma are stably active, and they have enhanced migratory capacity and secrete pro-tumorigenic growth factors and chemokines (3).
Immune cells in tumor microenvironment have a crucial role not only in malignant cell recognition and suppression but also in immunological escape. Macrophages can be polarized in two distinct phenotypes: anti-tumorigenic M1 producing reactive oxygen species and inflammatory cytokines, or pro-tumorigenic M2. Macrophages residing in an immunosuppressive tumor environment are usually shifted to a pro-tumorigenic subtype (M2) (4, 5). Tumor-infiltrating lymphocytes (TILs) can differentiate into many other different subtypes: CTLs, Th1, Th2, Th17, and Treg; among them, Tregs exert pro-tumorigenic activity. Myeloid-derived suppressor cells (MDSCs) are recruited in the tumor stroma from the bone marrow, where they mediate immunosuppressive functions as recruitment of Tregs (6–8).
New blood vessel formation is crucial for nutrient supply, waste removal, and metastatic dissemination: endothelial cells (ECs), together with pericytes, are responsible for angiogenesis and supporting tumor growth. Angiogenesis can be triggered not only by cancer cells in a hypoxic environment but also by stromal and infiltrating immune cells. Among the factors that promote angiogenesis, the hypoxia-inducible factor (HIF), the vascular endothelial growth factor (VEGF), and the platelet-derived growth factor (PDGF) play a key part (9). The role in tumor vascularization of pericytes is not completely understood, evidences underline a modulation of EC permeability, vessel stabilization, and metastasis together with degradation of endothelial ECM (10).
Collagens, proteoglycans and glycoproteins (laminins, elastin, fibronectin) are the main components of ECM. The ECM is a dynamic scaffold that provides structural organization and physical support to cells within the tissue, mediating also the release of growth factors and signaling molecules in a time- and context-dependent manner. Abnormal microenvironment can dysregulate the behavior of stromal cells in the TME and facilitate angiogenesis, inflammation, and tumor growth (11, 12).
In the last decade, studies on the microenvironment of the epithelial cancers, such as breast, lung, and colon tumors, with respect to sarcoma have been particularly developed. Indeed, the availability of samples together with the well-defined elements that constitute the microenvironment of the epithelial tumors allowed the achievement of a quite deep knowledge of the milieu with which cells interact (13–15). Consequently, the study of the altered, pro-tumorigenic environment has been considered as a new therapeutic approach to eradicate the disease. On the other hand, the sarcoma tumors are a quite heterogeneous group of mesenchymal malignancies (16). Patient-derived samples are mostly not available for research use, mainly because (i) sarcoma are rare and (ii) the small samples are devoted to pathologists and geneticists. For all these reasons, we thought that the data collection on what is the present knowledge on carcinoma vs. sarcoma microenvironment could be of interest for the scientific community. With both carcinoma and sarcoma occupying a wide field in cancer studies, we decided to start from our expertise, and for this minireview, we chose to consider colorectal cancer (CRC) and rhabdomyosarcoma (RMS) as peculiar examples of carcinoma and sarcoma, respectively.
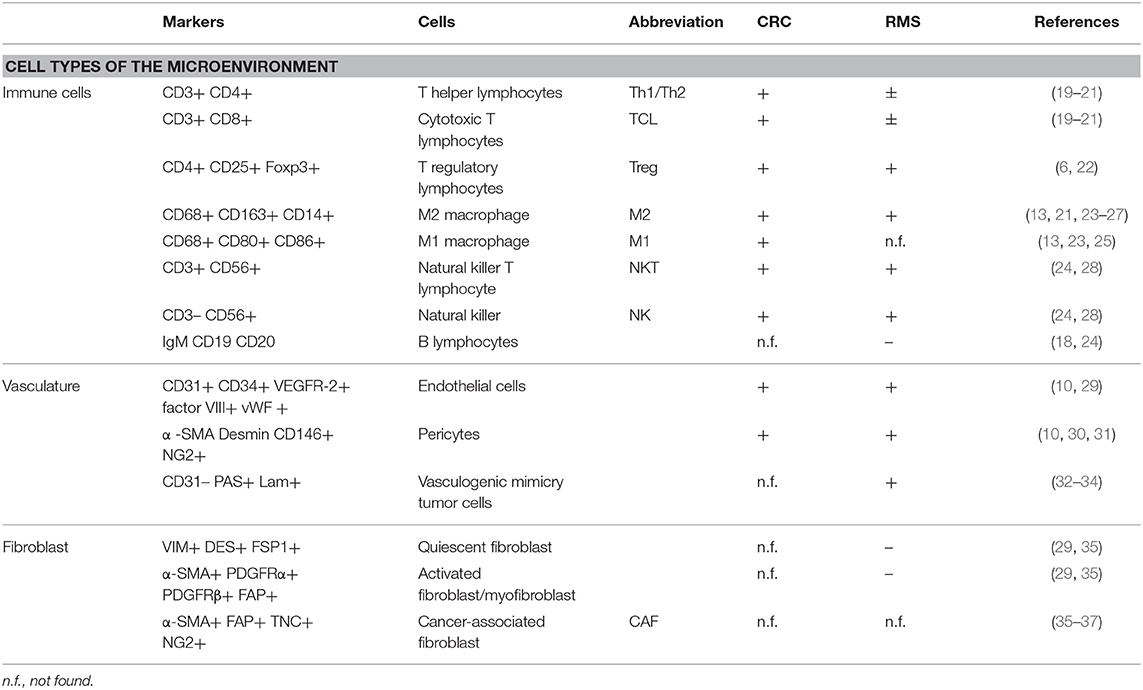
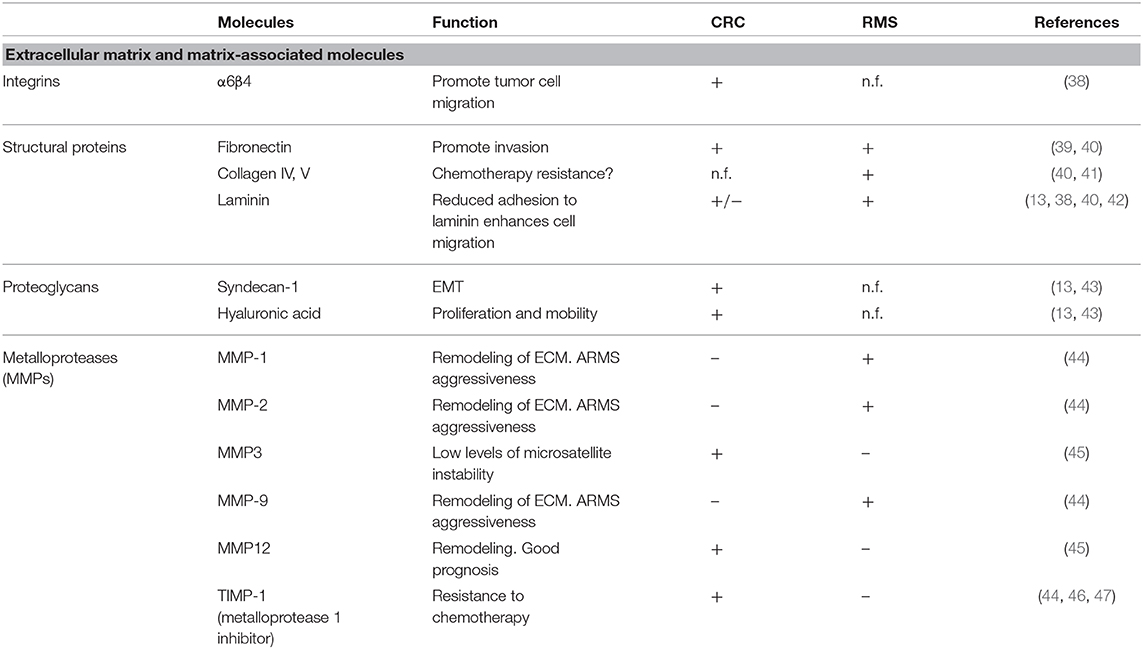
In this manuscript, we will focus on the microenvironment of CRC and RMS in order to draw the attention from a cell-centric description on the malignancies to a more complete overview on the TME. To underline the importance of microenvironment, a quite recent classification according to milieu composition has been proposed for CRC (17). A clearer understanding of TME elements, such as immune and stromal cells can help stratification of patients and the design of new approaches for personalized medicine (18). Tables 1, 2 summarize the CRC and RMS TME players.
Colorectal Carcinoma
Worldwide, CRC is the fourth most common cancer in both sexes and one of the major causes of mortality (48). The survival of CRC patients is highly correlated to the disease stage at diagnosis (49). CRC etiology is characterized by acquisition and progressive accumulation of genetic and epigenetic mutations by the somatic cells that confer a malignant phenotype.
However, only recently has the cell-centric view of tumor been replaced by a broader view that includes the complex TME in which the tumor cell grows, subsists, and co-evolves with it (50, 51).
Cellular Components of CRC
Cancer-Associated Fibroblast (CAF)
Fibroblasts are the major stromal population of healthy colon mucosa; following neoplastic transformation of the epithelial cells, fibroblasts become the major component of the tumor stroma and contribute to the maintenance of this malign state through a molecular mechanism based on an “efferent” and an “afferent” pathway (52). In the “efferent” pathway, cancer cells secrete soluble signals responsible for fibroblast differentiation into CAF (36, 53). On the other hand, CAFs along the “afferent” pathway affect tumor cells proliferation, migration, and invasion (30, 54). CAFs are the most abundant stromal population in CMS4 CRC subtype, suggesting a possible involvement in building highly vascularized and inflammatory tumors. In a study of Herrera and colleagues, it was demonstrated that co-cultivating CRC cell line with CAFs increases CRC cell line migration potential. In addition, they proposed a CAF gene expression profile and a CAF signature that clusters patients with CRC into high- and low-risk groups (55). In a further study, Berdiel-Acer et al. derived the transcription profile of healthy fibroblasts, CAFs from primary CRC, and CAFs from CRC liver metastasis. Finally, they obtained a 19-gene signature, able to predict tumor recurrence in two independent datasets (56).
Immune Cells
Since the second decade of the 1900s, the hypothesis that immune cells represent a host defense mechanism against cancer and that the abundance of immune infiltrates correlated with patients survival was formed (57). In particular, for CRC, the first evidence supporting this hypothesis was published at the end of the 1970s (57–59). Further studies led to the identification of the most important players in this complex mechanism; this category of cells acquired the name tumor-infiltrating lymphocyte (TIL), which is composed of T-lymphocytes, B-lymphocytes, macrophages, and natural killer cells (19, 22).
A study proposed by Suzuki et al. in CRC liver metastasis patients demonstrated that high peritumoral mast cell (MC) infiltration, positive to tryptase, predicts poor prognosis (60). A CRC with high density of MCs has been related to tumor aggressiveness and reduced survival. Finally, increased peritumoral infiltrating MCs have been demonstrated as a predictive marker of poor outcome in CRC liver metastasis patients.
Recently, it has been demonstrated that tumor-associated neutrophils (TANs) can be polarized by the tumor microenvironment into cells with an N1 or N2 phenotype. These phenotypical and molecular-different cells have anti-tumor or pro-tumor properties, respectively. The anti-tumor TAN-N1 actively expresses immunoactive cytokines and chemokines and decreased arginase expression (61). By contrast, the pro-tumoral TAN-N2 decreased the expression of inflammatory-promoting molecules and increased arginase expression, causing the inactivation of T cell effectors (13). Interestingly, in advanced CRC, the extent of the systemic immune response, revealed by the lymphocyte/neutrophils ratio, has been correlated to a poor outcome (62, 63).
Stromal cell subtypes that are predominant in CRC microenvironment are the tumor-associated macrophages (TAMs). In CRC, longer patient survival and metastasis absence are associated with anti-tumoral TAM infiltration. Conversely, poor prognosis and cancer progression are associated with pro-tumoral TAM infiltration (23). However, despite this rigid classification based on phenotypic and molecular characteristics, it is still controversial if M1 or M2 macrophages strictly exert an anti- or pro-tumor activity in CRC TME. In fact, the modulation of their activity seems to be localized within the tumor mass. TAMs at the tumor edges induce apoptosis in cancer cells, while TAMs localizing at the invasive front showed a resistant phenotype to suppressive TME (64, 65). Recent studies highlight that TAM plasticity, modulated by different microenvironmental factors, has a prognostic impact on CRC patients (64, 65). An integrative study, proposed by Mlecnik et al. showed that among the heterogeneous group of CRC patients with a mismatch repair-deficient and microsatellite instability-high (MSI-H) phenotype, two groups can be distinguished: the most represented group with high T-cell activity and improved prognosis, and a minor group with reduced T-cell activity and poor prognosis (66). Recent studies showed that the evaluation and characterization of TIL at the tumor site could be used also as predictive factor for the response to immunotherapy. In this context, a preliminary study proposed by Le et al. demonstrated that mismatch repair-deficient and hypermutated MSI-H metastatic CRC patients could significantly benefit from treatment with anti-PD-1 (67). After the seminal observation, they demonstrated that mismatch repair and microsatellite instability status were able to predict clinical benefits to anti-PD1 treatment in metastatic CRC patients (18). In a recent study, Overman et al. observed that single-immunotherapy treatment using anti-PD1 was more effective in the MSI-H metastatic CRC group compared with the non-MSI group (68). A similar result was reported with the double-immunotherapy treatment combining anti-PD1 with anti-CTLA4 (68).
Endothelial Cells
Angiogenesis in CRC is finely orchestrated by a series of vascular factors such as VEGF family proteins and receptors, placental growth factor (PLGF), and two neuropilin co-receptors (NRP1 and NRP2) (69, 70). At the CRC invasion front, a high vascular density correlates with tumor recurrence and metastasis (10). The increased expression of VEGFR-2 in CRC liver metastasis promotes the neo-vascular switch, increasing the nutrient supply fundamental for tumor progression (69). Furthermore, PlGF/VEGFR-1 signaling promotes CRC invasion through a p38-MMP9 pathway and is associated with a worse prognosis (71). CRC patients often present at systemic level tumor-derived ECs, expressing both epithelial and mesenchymal markers (72).
Finally, in a recent study, Lu et al. proposed ECs not only as a vessel-forming cells but they also demonstrated that ECs were able to activate the Notch signaling pathway in CRC cells through a paracrine secretion of Jagged-1, increasing their stemness and chemoresistance (73).
Acellular Components of CRC: ECM and Matrix-Associated Molecules
In order to progress and invade, cancer cells must overcome the physical barriers by interacting with ECM and its components. A study of Rabinovitz et al. demonstrated that in CRC cell lines, α6β4 integrin promotes cell migration by interacting with laminin-1 (38). Zapatka et al. demonstrated that the inactivation of tumor suppressor Smad4 gene, a late event on the carcinogenic chain of CRC, leads to a decreased expression of all three genes encoding laminin-5 that can favor invasion and metastasis spread (74). Ding et al. demonstrated that fibronectin, a high-molecular-weight adhesive glycoprotein present in the ECM, can promote invasion of colon cells via an up-regulation of focal adhesion kinase (39). Proteoglycans are a subset of molecules altered in CRC. Hashimoto et al., showed that basolateral border expression of syndecan-1 is associated with normal colonic epithelial cells, while in CRC, its expression is completely lost and this has been correlated with malignancy, epithelial–mesenchymal transition, tumor-node-metastasis stage, and local lymph node metastasis (43). Glycosaminoglycans are another key ECM component frequently deregulated in cancer. In CRC, hyaluronic acid (HA) enhances cell proliferation and motility in vitro and in vivo (42, 75). Dunn et al. showed that the inhibition of HA production in SW620 blocks in vitro Matrigel invasion (76). In HCT-116 cells, the interaction of HA with CD44 stimulates cell survival, proliferation, adhesion, and invasion through ERBB2 activation (77, 78).
The proteinases that regulate ECM turnover and remodeling are another intriguing component of ECM. Zucker et al. demonstrated that matrix metalloproteases (MMPs) are correlated with tumor stage and prognosis. In this context, the MMPE up-regulation correlates with MSI-L and bad prognosis. Conversely, overexpression of MMP12 is associated with a better prognosis in CRC (45). Davidsen et al. demonstrated that CRC cells actively expressing TIMP-1 protein showed an increased resistance to drugs compared to TIMP-1 silenced cells (46). In line with this study, Sorensen et al. showed that high TIMP-1 level in CRC tissue and plasma correlated with a bad prognosis (47).
Rhabdomyosarcoma
Among the tumors of mesenchymal origin, RMS is the most common soft tissue sarcoma in children and young adults with an incidence of 4.5 cases among 1,000,000 newborns. The two main subtypes are the embryonal rhabdomyosarcoma (ERMS) and alveolar rhabdomyosarcoma (ARMS), accounting, respectively, for the 57% and the 23% of all diagnosed RMS (79). ERMS is associated with a better prognosis and higher relative 5-year survival rates (73.4%). ARMS is associated with poorer outcome and a lower 5-year survival rate (47.8%) due to the high aggressiveness and tendency to metastasize (79, 80). Following the guidelines of the European Pediatric Soft Tissue Sarcoma Study Group (EpSGG) for RMS 2005 protocol, patients diagnosed with RMS were stratified in four risk groups: low, standard, high, and very high risk. Prognostic factors considered are: pathology (favorable for embryonal, spindle cells and botryoid RMS and unfavorable for ARMS), post-surgical stage (from complete resection to macroscopic residual), site of onset, lymph node involvement, size of the mass, and age of the patient (81). Similarly, the guidelines for RMS patient stratification given by the Children's Oncology Group identify four risk categories (low risk subset 1, low risk subset 2, intermediate risk, and high risk) considering histology, site of onset, size, nodal involvement, presence of distant metastases, and Intergroup Rhabdomyosarcoma Study classification based on residual disease after surgery (82). In both protocols, stromal cell population and the TME are not considered for diagnostic purposes.
Cellular Components of RMS
Cancer-Associated Fibroblast (CAF)
The role of fibroblasts in RMS has not been precisely investigated yet. RMS cell lines express Macrophage migration Inhibitory Factor (MIF). An interesting result obtained by Tarnowski and colleagues demonstrate that MIF, interacting with RMS cell surface receptors CXCR4 and CXCR7 in a paracrine loop, increases cell adhesion, vascularization, and reduces the number of infiltrating CAF. Down-regulation of MIF in the RMS cell line, used for xenograft production, resulted in bigger sized xenografts, higher stromal cell support, and a higher number of circulating tumor cells (37). The presence of a stromal compartment in sarcomas has been questioned in the study of Tomlinson et al., where the difference in the pattern of blood vessels distribution in sarcoma and carcinoma tumor masses has been attributed to the presence of fibroblasts and myofibroblasts in the latter, and the absence of these cells in the former (29).
Immune Cells
The presence of the immune compartment in RMS is still debated. D'Angelo and colleagues selected a cohort of 50 patients with soft tissue sarcomas to examine the immune milieu. CD3+ (TILs), CD4+ (T-helper cells), CD8+ (cytotoxic T-cells), and FOXP3+ (Treg) lymphocytes were found in 98% of the biopsies, while macrophages were found in 90% of the cases. The lower presence of CD3+− and CD4+− infiltrating lymphocytes correlates with a favorable outcome (20), in contrast with a larger dataset of different tumors showing a positive correlation between CD3+ and CD4+ infiltrates and survival (83). Higher number of CD8+ cells were found in patients with larger tumors or with metastasis (20). However, this study presents some critical limitations: the low number of tumor specimens representing each histological subtype (20 different subtypes represented by 1 or 2 specimens each) and samples representing the same malignancy but with different stages of the disease. A recent work divided a cohort of 25 RMS (13 embryonal, 11 alveolar, and 1 sclerosing) into 4 categories based on the expression of PD-L1. Although RMS cells were PD-L1−, immune infiltrating cells (CD3+ lymphocytes and CD68+ macrophages) were found in 6/11 and 9/14 of ARMS and ERMS specimens, respectively, with different patterns and grades of PD-L1 positivity. PD-L1-positive immune cells were observed within the tumor burden in 4/25 of the cases, in the surrounding stroma in 5/25 of the cases, and finally in 7/25 of the specimens, very few T-cells in either the parenchyma or the stroma could be found. However, due to the limited size of the cohort, authors could not identify any correlation between PD-L1 status and RMS subtypes or the clinical outcome (24). Regarding the relation between RMS cells and the immune system in vitro, it is shown that cytotoxic drugs such as doxorubicin provoke the translocation of calreticulin from the endoplasmic reticulum to the cell surface and, in combination with anti-PD-L1 antibody, increase the efficiency of phagocytosis by macrophages (84). Moreover, doxorubicin increases MIF expression in RMS cell lines. MIF induces the differentiation of pro-tumorigenic CD33+ CD14+ myeloid-derived suppressor cells (MDSCs) from peripheral blood mononuclear cells (PBMCs). However, inhibition of MIF impairs the migration potential of RMS cells (85), in contrast with the results obtained in the work previously mentioned (37).
Endothelial Cells
The presence of ECs and evidences of vascularization in soft tissue sarcomas (STS) were investigated by West and colleagues who evaluated the microvessel density (MVD) in 42 high-grade STS patients with different histology. Diffuse staining of VEGF was reported in 41/42 samples but without any correlation with microvessel density. Hot spots of angiogenesis were found in only 33% of the tissue specimens, highlighting a homogeneous microvessel distribution that, however, did not correlate with survival of the patients or presence of metastasis (86). The main signals promoting angiogenesis in RMS are hypoxia, VEGF (87), and PDGF (88, 89). To stimulate angiogenesis, VEGF, expressed in all the cell lines tested, and its receptors, in particular VEGFR-1 that is present in 4/6 of the cell lines considered, sustain an autocrine positive feedback-loop in RMS cell lines, promoting proliferation, and cell growth (90). VEGF is expressed more frequently in ARMS (70.6%) than in ERMS (50.0%) and its expression is associated to poor prognosis for both subtypes, representing a valuable potential therapeutic target (87). An alternative, or complementary mechanism for blood diffusion within the tumor is the vasculogenic mimicry (VM): some tumor cells express endothelium-associated genes and form loops and arc networks rich in laminin lined with other tumor cells. These “channel-like spaces” provide a blood perfusion and a dissemination route (32). Two different studies highlight the vasculogenic mimicry mechanism in sarcomas: the first consider a cohort of 32 patients with orbital RMS, showing a strong correlation between VM and poor outcome (33). The latter analyzes a larger cohort of patients showing positive correlation between VM and poorer outcome in mesothelial sarcoma and ARMS patients (34).
Acellular Components of RMS: ECM and Matrix-Associated Molecules
The characterization of RMS ECM itself is still incomplete. Scarpa and colleagues, in 1987, studied in vitro the matrix composition of different pediatric tumor cell lines, and they reported high variability between RMS subtype considered (alveolar, embryonal, unclassified, and synovial sarcoma). ARMS and ERMS produce different matrices: the former synthetize mainly collagen V and laminin and the latter almost exclusively collagen IV and fibronectin. In both cases, deposition of collagen I and III, interstitial collagens typical of mesenchymal cells, was not reported (40). Interactions between ECM and RMS cells are also altered: the expression of α-dystroglican, important complex for laminin and basement membrane assembly and binding, is down-regulated in RMS and other pediatric solid tumors. Even if the implication of this differential expression in tumor biology has yet to be clarified, reduced adhesion to laminin of these cells can result in enhanced migration ability (91). The matrix degradation in the tumor environment is fundamental for tumor development and invasiveness, releasing growth factors, guiding cell migration, and promoting angiogenesis. Of particular interest is that remodeling enzymes MMP-1, MMP-2, and MMP-9 are found to be up-regulated in ARMS compared to ERMS, and this can be one of the factors responsible for the higher aggressiveness of the alveolar subtype (44).
Concluding Remarks
Drawing the attention toward the microenvironment, the epithelial origin of carcinoma characterizes the development of a microenvironment with common cellular and non-cellular elements that can be found in colon, lung, and breast (13–15). On the other hand, sarcomas are rare cancers of mesenchymal origin and are characterized by high heterogeneity of the histologies and genetic and clinical features. In the past, sarcoma and, in particular, RMS were believed “one-compartment tumors” with mainly cancer cells (29). Now it is more clear that, although at a lower level with respect to carcinoma, the stromal cell heterogeneity is present and it is worth studying it (92). Indeed, the multifaceted nature of the TME has an impact on tumor progression, as both tumor and stromal cells change during time, developing angiogenic, invasive, and migratory properties. Common targets for therapeutic approaches of CRC and RMS are focused on antiangiogenic or immunomodulating effects, although the still poor knowledge of RMS TME makes this aspect difficult to develop. While significant progresses have been made for the study of TME in CRC, to date, precise characterization of the tumor stroma for soft tissue sarcomas, and particularly for RMS, is still lacking and urgently needed. It is conceivable that the cancer cells that survived also after chemotherapy are stem cells protected by the surrounding microenvironment where the stem cell niche is still intact. In this perspective, a deeper characterization of the microenvironment, and specifically for RMS, will help to identify (i) the mechanisms of tumor growth, development, and metastasis; (ii) how the crosstalk between cells in the stroma participate to the tumor progression; and finally (iii) new microenvironment-related therapeutic targets. These, in combination with present therapies, could open new avenues to ameliorate the overall survival of the patients.
Author Contributions
MS and ED'A wrote the article. GB and MA supervised the work. MP wrote the article, supervised, and gave the final approval.
Funding
This work has been supported by Progetto di Ateneo 2016, Padova University, by LIFELAB Program (Veneto Region) and AIRC Investigator Grant Id 19104, and Università degli Studi di Padova, Budget Integrato per la Ricerca dei Dipartimenti: (BIRD199592).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
ED'A was partially supported by LIFELAB Program (Veneto Region) and Italian Association for Cancer Research (AIRC). MP was funded by University of Padova, Grant number GRIC15AIPF, Assegno di Ricerca Senior.
References
1. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. (2012) 21:309–22. doi: 10.1016/j.ccr.2012.02.022
2. Darby IA, Laverdet B, Bonté F, Desmouliere A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. (2014) 7:301–11. doi: 10.2147/CCID.S50046
3. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. (2016) 16:582–98. doi: 10.1038/nrc.2016.73
4. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. (2017) 14:399–416. doi: 10.1038/nrclinonc.2016.217
5. Cook J, Hagemann T. Tumour-associated macrophages and cancer. Curr Opin Pharmacol. (2013) 13:595–601. doi: 10.1016/j.coph.2013.05.017
6. Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. (2008) 27:5904–12. doi: 10.1038/onc.2008.271
7. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
8. Shiao SL, Ganesan AP, Rugo HS, Coussens LM. Immune microenvironments in solid tumors: new targets for therapy. Genes Dev. (2011) 25:2559–72. doi: 10.1101/gad.169029.111
9. Mittal K, Ebos J, Rini B. Angiogenesis and the tumor microenvironment: vascular endothelial growth factor and beyond. Semin Oncol. (2014) 41:235–51. doi: 10.1053/j.seminoncol.2014.02.007
10. Raza A, Franklin MJ, Dudek AZ. Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am J Hematol. (2010) 85:593–8. doi: 10.1002/ajh.21745
11. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. (2014) 15:786–801. doi: 10.1038/nrm3904
12. Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. (2012) 196:395–406. doi: 10.1083/jcb.201102147
13. Peddareddigari VG, Wang D, Dubois RN. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron. (2010) 3:149–66. doi: 10.1007/s12307-010-0038-3
14. Altorki NK, Markowitz GJ, Gao D, Port JL, Saxena A, Stiles B, et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer. (2019) 19:9–31. doi: 10.1038/s41568-018-0081-9
15. Place AE, Jin Huh S, Polyak K. The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res. (2011) 13:227. doi: 10.1186/bcr2912
16. Genadry KC, Pietrobono S, Rota R, Linardic CM. Soft tissue sarcoma cancer stem cells: an overview. Front Oncol. (2018) 8:1–17. doi: 10.3389/fonc.2018.00475
17. Becht E, de Reyniès A, Giraldo NA, Pilati C, Buttard B, Lacroix L, et al. Immune and stromal classification of colorectal cancer is associated with molecular subtypes and relevant for precision immunotherapy. Clin Cancer Res. (2016) 22:4057–66. doi: 10.1158/1078-0432.CCR-15-2879
18. Koi M, Carethers JM. The colorectal cancer immune microenvironment and approach to immunotherapies. Futur Oncol. (2017) 13:1633–47. doi: 10.2217/fon-2017-0145
19. Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. (2013) 14:1014–22. doi: 10.1038/ni.2703
20. D'Angelo SP, Shoushtari AN, Agaram NP, Kuk D, Qin L-X, Carvajal RD, et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum Pathol. (2015) 46:357–65. doi: 10.1016/j.humpath.2014.11.001
21. Calì B, Molon B, Viola A. Tuning cancer fate: the unremitting role of host immunity. Open Biol. (2017) 7:170006. doi: 10.1098/rsob.170006
22. Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, et al. High frequency of tumor-infiltrating FOXP3 + regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. (2010) 126:2635–43. doi: 10.1002/ijc.24989
23. Erreni M, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) and inflammation in colorectal cancer. Cancer Microenviron. (2011) 4:141–54. doi: 10.1007/s12307-010-0052-5
24. Bertolini G, Bergamaschi L, Ferrari A, Renne SL, Collini P, Gardelli C, et al. PD-L1 assessment in pediatric rhabdomyosarcoma: a pilot study. BMC Cancer. (2018) 18:652. doi: 10.1186/s12885-018-4554-8
25. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. (2014) 13:1–13. doi: 10.12703/P6-13
26. Ehnman M, Larsson O. Microenvironmental targets in sarcoma. Front Oncol. (2015) 5:248. doi: 10.3389/fonc.2015.00248
27. Fujiwara T, Fukushi J, Yamamoto S, Matsumoto Y, Setsu N, Oda Y, et al. Macrophage infiltration predicts a poor prognosis for human ewing sarcoma. Am J Pathol. (2011) 179:1157–70. doi: 10.1016/j.ajpath.2011.05.034
28. Paunescu V, Bojin FM, Tatu CA, Gavriliuc OI, Rosca A, Gruia AT, et al. Tumour-associated fibroblasts and mesenchymal stem cells: more similarities than differences. J Cell Mol Med. (2011) 15:635–46. doi: 10.1111/j.1582-4934.2010.01044.x
29. Tomlinson J, Barsky SH, Nelson S, Singer S, Pezeshki B, Lee MC, et al. Different patterns of angiogenesis in sarcomas and carcinomas. Clin Cancer Res. (1999) 5:3516–22.
30. Colangelo T, Polcaro G, Muccillo L, D'Agostino G, Rosato V, Ziccardi P, et al. Friend or foe? The tumour microenvironment dilemma in colorectal cancer. Biochim Biophys Acta Rev cancer. (2016) 1867:1–18. doi: 10.1016/j.bbcan.2016.11.001
31. Chang L, Nguyen V, Nguyen A, Scott MA, James AW. Pericytes in sarcomas of bone. Med Oncol. (2015) 32:202. doi: 10.1007/s12032-015-0651-6
32. Hendrix MJC, Seftor EA, Hess AR, Seftor REB. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. (2003) 3:411–21. doi: 10.1038/nrc1092
33. Chen L, He Y, Sun S, Sun B, Tang X. Vasculogenic mimicry is a major feature and novel predictor of poor prognosis in patients with orbital rhabdomyosarcoma. Oncol Lett. (2015) 10:1635–41. doi: 10.3892/ol.2015.3469
34. Sun B, Zhang S, Zhao X, Zhang W, Hao X. Vasculogenic mimicry is associated with poor survival in patients with mesothelial sarcomas and alveolar rhabdomyosarcomas. Int J Oncol. (2004) 25:1609–14. doi: 10.3892/ijo.25.6.1609
35. Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. (2006) 6:392–401. doi: 10.1038/nrc1877
36. Hawinkels LJ, Paauwe M, Verspaget HW, Wiercinska E, Van Der Zon JM, Van Der Ploeg K, et al. Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts. Oncogene. (2014) 33:97–107. doi: 10.1038/onc.2012.536
37. Tarnowski M, Grymula K, Liu R, Tarnowska J, Drukala J, Ratajczak J, et al. Macrophage migration inhibitory factor is secreted by rhabdomyosarcoma cells, modulates tumor metastasis by binding to CXCR4 and CXCR7 receptors and inhibits recruitment of cancer-associated fibroblasts. Mol Cancer Res. (2010) 8:1328–43. doi: 10.1158/1541-7786.MCR-10-0288
38. Rabinovitz I, Mercurio AM. The integrin α6β4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J Cell Biol. (1997) 139:1873–84. doi: 10.1083/jcb.139.7.1873
39. Ding J, Li D, Wang X, Wang C, Wu T. Fibronectin promotes invasiveness and focal adhesion kinase tyrosine phosphorylation of human colon cancer cell. Hepatogastroenterology. (2008) 55:2072–6.
40. Scarpa S, Modesti A, Triche TJ. Extracellular matrix synthesis by undifferentiated childhood tumor cell lines. Am J Pathol. (1987) 129:74–85.
41. Kirkland SC. Type I collagen inhibits differentiation and promotes a stem cell-like phenotype in human colorectal carcinoma cells. Br J Cancer. (2009) 101:320–6. doi: 10.1038/sj.bjc.6605143
42. Laurich C, Wheeler MA, Iida J, Neudauer CL, McCarthy JB, Bullard KM. Hyaluronan mediates adhesion of metastatic colon carcinoma cells. J Surg Res. (2004) 122:70–4. doi: 10.1016/j.jss.2004.05.018
43. Hashimoto Y, Skacel M, Adams JC. Association of loss of epithelial syndecan-1 with stage and local metastasis of colorectal adenocarcinomas: an immunohistochemical study of clinically annotated tumors. BMC Cancer. (2008) 8:1–7. doi: 10.1186/1471-2407-8-185
44. Diomedi-Camassei F, Boldrini R, Ravà L, Donfrancesco A, Boglino C, Messina E, et al. Different pattern of matrix metalloproteinases expression in alveolar versus embryonal rhabdomyosarcoma. J Pediatr Surg. (2004) 39:1673–9. doi: 10.1016/j.jpedsurg.2004.07.014
45. Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. (2004) 23:101–17. doi: 10.1023/A:1025867130437
46. Davidsen ML, Würtz S, Rømer MU, Sørensen NM, Johansen SK, Christensen IJ, et al. TIMP-1 gene deficiency increases tumour cell sensitivity to chemotherapy-induced apoptosis. Br J Cancer. (2006) 95:1114–20. doi: 10.1038/sj.bjc.6603378
47. Sorensen NM, Bystrom P, Christensen IJ, Berglund A, Nielsen HJ, Brunner N, et al. TIMP-1 is significantly associated with objective response and survival in metastatic colorectal cancer patients receiving combination of irinotecan, 5-fluorouracil, and folinic acid. Clin Cancer Res. (2007) 13:4117–22. doi: 10.1158/1078-0432.CCR-07-0186
48. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
49. Haggar F, Boushey R. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. (2009) 22:191–7. doi: 10.1055/s-0029-1242458
50. Crotti S, Piccoli M, Rizzolio F, Giordano A, Nitti D, Agostini M. Extracellular matrix and colorectal cancer: how surrounding microenvironment affects cancer cell behavior? J Cell Physiol. (2017) 232:967–75. doi: 10.1002/jcp.25658
51. D'Angelo E, Agostini M. Long non-coding RNA and extracellular matrix: the hidden players in cancer-stroma cross-talk. Non Coding RNA Res. (2018) 3:174–7. doi: 10.1016/j.ncrna.2018.08.002
52. Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res. (2012) 10:1403–18. doi: 10.1158/1541-7786.MCR-12-0307
53. Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res. (2011) 1:482–97.
54. Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M, et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. (2014) 14:342–56. doi: 10.1016/j.stem.2014.01.009
55. Herrera M, Islam AB, Herrera A, Martín P, García V, Silva J, et al. Functional heterogeneity of cancer-associated fibroblasts from human colon tumors shows specific prognostic gene expression signature. Clin Cancer Res. (2013) 19:5914–26. doi: 10.1158/1078-0432.CCR-13-0694
56. Berdiel-Acer M, Cuadras D, Diaz-Maroto NG, Sanjuan X, Serrano T, Berenguer A, et al. A monotonic and prognostic genomic signature from fibroblasts for colorectal cancer initiation, progression, and metastasis. Mol Cancer Res. (2014) 12:1254–66. doi: 10.1158/1541-7786.MCR-14-0121
57. Underwood JC. Lymphoreticular infiltration in human tumours: prognostic and biological implications: a review. Br J Cancer. (1974) 30:538–48. doi: 10.1038/bjc.1974.233
58. Murray D, Hreno A, Dutton J, Hampson LG. Prognosis in colon cancer: a pathologic reassessment. Arch Surg. (1975) 110:908–13. doi: 10.1001/archsurg.1975.01360140052011
59. Watt AG, House AK. Colonic carcinoma: a quantitative assessment of lymphocyte infiltration at the periphery of colonic tumors related to prognosis. Cancer. (1978) 41:279–82. doi: 10.1002/1097-0142(197801)41:1<279::AID-CNCR2820410139>3.0.CO;2-B
60. Suzuki S, Ichikawa Y, Nakagawa K, Kumamoto T, Mori R, Matsuyama R, et al. High infiltration of mast cells positive to tryptase predicts worse outcome following resection of colorectal liver metastases. BMC Cancer. (2015) 15:1–8. doi: 10.1186/s12885-015-1863-z
61. Granot Z, Jablonska J. Distinct functions of neutrophil in cancer and its regulation. Mediat Inflamm. (2015) 2015: 701067. doi: 10.1155/2015/701067
62. Arelaki S, Arampatzioglou A, Kambas K, Papagoras C, Miltiades P, Angelidou I, et al. Gradient infiltration of neutrophil extracellular traps in colon cancer and evidence for their involvement in tumour growth. PLoS ONE. (2016) 11:e0154484. doi: 10.1371/journal.pone.0154484
63. Pine JK, Morris E, Hutchins GG, West NP, Jayne DG, Quirke P, et al. Systemic neutrophil-to-lymphocyte ratio in colorectal cancer: the relationship to patient survival, tumour biology and local lymphocytic response to tumour. Br J Cancer. (2015) 113:204–11. doi: 10.1038/bjc.2015.87
64. Koelzer VH, Canonica K, Dawson H, Sokol L, Karamitopoulou-Diamantis E, Lugli A, et al. Phenotyping of tumor-associated macrophages in colorectal cancer: impact on single cell invasion (tumor budding) and clinicopathological outcome. Oncoimmunology. (2016) 5:1–10. doi: 10.1080/2162402X.2015.1106677
65. Norton SE, Dunn ETJ, McCall JL, Munro F, Kemp RA. Gut macrophage phenotype is dependent on the tumor microenvironment in colorectal cancer. Clin Transl Immunol. (2016) 5:e76. doi: 10.1038/cti.2016.21
66. Mlecnik B, Bindea G, Kirilovsky A, Angell HK, Obenauf AC, Tosolini M, et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med. (2016) 8:327ra26. doi: 10.1126/scitranslmed.aad6352
67. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. (2015) 372:2509–20. doi: 10.1200/jco.2015.33.15_suppl.lba100
68. Overman MJ, Kopetz S, McDermott RS, Leach J, Lonardi S, Lenz H-J, et al. Nivolumab ± ipilimumab in treatment (tx) of patients. (pts) with metastatic colorectal cancer (mCRC) with and without high microsatellite instability (MSI-H): checkMate-142 interim results. J Clin Oncol. (2016) 34:3501. doi: 10.1200/JCO.2016.34.15_suppl.3501
69. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
70. Mousa L, Salem ME, Mikhail S. Biomarkers of angiogenesis in colorectal cancer. Biomark Cancer. (2015) 7:13–9. doi: 10.4137/BIC.S25250
71. Wei SC, Tsao PN, Weng MT, Cao Z, Wong JM. Flt-1 in colorectal cancer cells is required for the tumor invasive effect of placental growth factor through a p38-MMP9 pathway. J Biomed Sci. (2013) 20:39. doi: 10.1186/1423-0127-20-39
72. Cima I, Kong SL, Sengupta D, Tan IB, Phyo WM, Lee D, et al. Tumor-derived circulating endothelial cell clusters in colorectal cancer. Sci Transl Med. (2016) 8:345ra89. doi: 10.1126/scitranslmed.aad7369
73. Lu J, Ye X, Fan F, Xia L, Bhattacharya R, Bellister S, et al. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell. (2013) 23:171–85. doi: 10.1016/j.ccr.2012.12.021
74. Zapatka M, Zboralski D, Radacz Y, Böckmann M, Arnold C, Schöneck A, et al. Basement membrane component laminin-5 is a target of the tumor suppressor Smad4. Oncogene. (2007) 26:1417–27. doi: 10.1038/sj.onc.1209918
75. Kim HR, Wheeler MA, Wilson CM, Iida J, Eng D, Simpson MA, et al. Hyaluronan facilitates invasion of colon carcinoma cells in vitro via interaction with CD44. Cancer Res. (2004) 64:4569–76. doi: 10.1158/0008-5472.CAN-04-0202
76. Bullard Dunn KM, Lee PK, Wilson CM, Iida J, Wasiluk KR, Hugger M, et al. Inhibition of hyaluronan synthases decreases matrix metalloproteinase-7 (MMP-7) expression and activity. Surgery. (2009) 145:322–9. doi: 10.1016/j.surg.2008.11.008
77. Misra S, Toole BP, Ghatak S. Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J Biol Chem. (2006) 281:34936–41. doi: 10.1074/jbc.C600138200
78. Ghatak S, Misra S, Toole BP. Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells. J Biol Chem. (2005) 280:8875–83. doi: 10.1074/jbc.M410882200
79. Ognjanovic S, Linabery AM, Charbonneau B, Ross JA. Trends in childhood rhabdomyosarcoma incidence and survival in the United States, 1975-2005. Cancer. (2009) 115:4218–26. doi: 10.1002/cncr.24465
80. Meza JL, Anderson J, Pappo AS, Meyer WH. Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on intergroup rhabdomyosarcoma studies III and IV: the children's oncology group. J Clin Oncol. (2006) 24:3844–51. doi: 10.1200/JCO.2005.05.3801
81. EPSSG RMS 2005: A Protocol for Non Metastatic Rhabdomyosarcoma. (2008). Available online at: https://www.skion.nl/workspace/uploads/Protocol-EpSSG-RMS-2005-1-3-May-2012_1.pdf
82. Malempati S, Hawkins DS. Rhabdomyosarcoma: review of the children's oncology group (COG) soft-tissue Sarcoma committee experience and rationale for current COG studies. Pediatr Blood Cancer. (2012) 59:5–10. doi: 10.1002/pbc.24118
83. Gooden MJM, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. (2011) 105:93–103. doi: 10.1038/bjc.2011.189
84. Herrmann D, Seitz G, Fuchs J, Armeanu-Ebinger S. Susceptibility of rhabdomyosarcoma cells to macrophage-mediated cytotoxicity. Oncoimmunology. (2012) 1:279–86. doi: 10.4161/onci.18612
85. Johler SM, Fuchs J, Seitz G, Armeanu-Ebinger S. Macrophage migration inhibitory factor (MIF) is induced by cytotoxic drugs and is involved in immune escape and migration in childhood rhabdomyosarcoma. Cancer Immunol Immunother. (2016) 65:1465–76. doi: 10.1007/s00262-016-1896-4
86. West CC, Brown NJ, Mangham DC, Grimer RJ, Reed MWR. Microvessel density does not predict outcome in high grade soft tissue sarcoma. Eur J Surg Oncol. (2005) 31:1198–205. doi: 10.1016/j.ejso.2005.04.012
87. Miyoshi K, Kohashi K, Fushimi F, Yamamoto H, Kishimoto J, Taguchi T, et al. Close correlation between CXCR4 and VEGF expression and frequent CXCR7 expression in rhabdomyosarcoma. Hum Pathol. (2014) 45:1900–9. doi: 10.1016/j.humpath.2014.05.012
88. Ehnman M, Missiaglia E, Folestad E, Selfe J, Strell C, Thway K, et al. Distinct effects of ligand-induced PDGFR and PDGFR signaling in the human rhabdomyosarcoma tumor cell and stroma cell compartments. Cancer Res. (2013) 73:2139–49. doi: 10.1158/0008-5472.CAN-12-1646
89. Taniguchi E, Nishijo K, McCleish AT, Michalek JE, Grayson MH, Infante AJ, et al. PDGFR-A is a therapeutic target in alveolar rhabdomyosarcoma. Oncogene. (2008) 27:6550–60. doi: 10.1038/onc.2008.255
90. Gee MFW, Tsuchida R, Eichler-Jonsson C, Das B, Baruchel S, Malkin D. Vascular endothelial growth factor acts in an autocrine manner in rhabdomyosarcoma cell lines and can be inhibited with all-trans-retinoic acid. Oncogene. (2005) 24:8025–37. doi: 10.1038/sj.onc.1208939
91. Martin LT, Glass M, Dosunmu E, Martin PT. Altered expression of natively glycosylated α dystroglycan in pediatric solid tumors. Hum Pathol. (2007) 38:1657–68. doi: 10.1016/j.humpath.2007.03.025
Keywords: tumor microenvironment, colon rectal cancer, rhabdomyosarcoma, extracellular matrix, microenvironment remodeling
Citation: Saggioro M, D'Angelo E, Bisogno G, Agostini M and Pozzobon M (2020) Carcinoma and Sarcoma Microenvironment at a Glance: Where We Are. Front. Oncol. 10:76. doi: 10.3389/fonc.2020.00076
Received: 02 August 2019; Accepted: 15 January 2020;
Published: 03 March 2020.
Edited by:
Rimas J. Orentas, Seattle Children's Research Institute, United StatesReviewed by:
Rossella Rota, Bambino Gesù Children Hospital (IRCCS), ItalyEleanor Chen, University of Washington, United States
Copyright © 2020 Saggioro, D'Angelo, Bisogno, Agostini and Pozzobon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Agostini, m.agostini@unipd.it; Michela Pozzobon, m.pozzobon@irpcds.org; michela.pozzobon@unipd.it
†These authors share first authorship
 Mattia Saggioro
Mattia Saggioro Edoardo D'Angelo
Edoardo D'Angelo Gianni Bisogno2
Gianni Bisogno2 Marco Agostini
Marco Agostini Michela Pozzobon
Michela Pozzobon