Water Structures and Packing Efficiency in Methylene Blue Cyanometallate Salts
Abstract
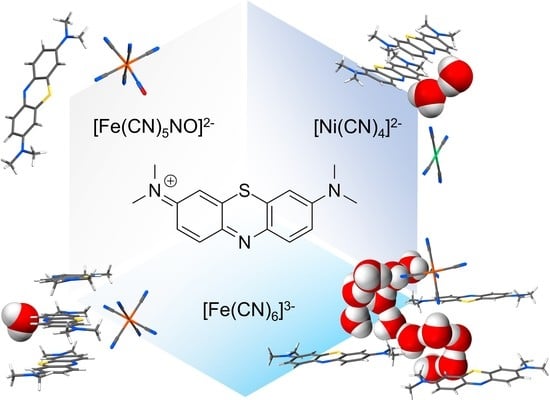
:1. Introduction
2. Materials and Methods
2.1. General Remarks
2.2. Synthetic Procedures
2.2.1. Synthesis of MB2[Ni(CN)4]·2H2O
2.2.2. Synthesis of MB2[Fe(CN)5NO]
2.2.3. Synthesis of MB3[Fe(CN)6]·H2O and MB3[Fe(CN)6]·12.36H2O
2.3. Single-Crystal X-ray Diffraction Analysis
2.4. Computational Details
3. Results and Discussion
3.1. Systematic Variation of Cyanometallate Ions
3.2. Crystal Structure Analysis
3.3. Packing Considerations
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kalaitzakis, D.; Kouridaki, A.; Noutsias, D.; Montagnon, T.; Vassilikogiannakis, G. Methylene Blue as a Photosensitizer and Redox Agent: Synthesis of 5-Hydroxy-1 H -pyrrol-2(5 H )-ones from Furans. Angew. Chem. Int. Ed. 2015, 54, 6283–6287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, R.; Najeeb, J.; Sattar, A.; Naseem, K.; Irfan, A.; Al-Sehemi, A.G.; Farooqi, Z.H. Chemical reduction of methylene blue in the presence of nanocatalysts: A critical review. Rev. Chem. Eng. 2019. [Google Scholar] [CrossRef]
- Schirmer, R.H.; Adler, H.; Pickhardt, M.; Mandelkow, E. “Lest we forget you - methylene blue…”. Neurobiol. Aging 2011, 32, 2325.e7–2325.e16. [Google Scholar] [CrossRef] [PubMed]
- Ginimuge, P.R.; Jyothi, S.D. Methylene blue: Revisited. J. Anaesthesiol. Clin. Pharmacol. 2010, 26, 517–520. [Google Scholar] [PubMed]
- Lo, J.C.Y.; Darracq, M.A.; Clark, R.F. A review of methylene blue treatment for cardiovascular collapse. Can. J. Emergency Med. Canadian 2014, 46, 670–679. [Google Scholar] [CrossRef]
- Oz, M.; Lorke, D.E.; Hasan, M.; Petroianu, G.A. Cellular and molecular actions of Methylene Blue in the nervous system. Med. Res. Rev. 2011, 31, 93–117. [Google Scholar] [CrossRef] [Green Version]
- Gutter, B.; Speck, W.T.; Rosenkranz, H.S. A study of the photoinduced mutagenicity of methylene blue. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 1977, 44, 177–181. [Google Scholar] [CrossRef]
- Li, R.; Chen, J.; Cesario, T.C.; Wang, X.; Yuan, J.S.; Rentzepis, P.M. Synergistic reaction of silver nitrate, silver nanoparticles, and methylene blue against bacteria. Proc. Natl. Acad. Sci. USA 2016, 113, 13612–13617. [Google Scholar] [CrossRef] [Green Version]
- Canossa, S.; Bacchi, A.; Graiff, C.; Pelagatti, P.; Predieri, G.; Ienco, A.; Manca, G.; Mealli, C. Hierarchy of Supramolecular Arrangements and Building Blocks: Inverted Paradigm of Crystal Engineering in the Unprecedented Metal Coordination of Methylene Blue. Inorg. Chem. Front. 2017, 56, 3512–3516. [Google Scholar] [CrossRef]
- Wood, P.A.; Olsson, T.S.G.; Cole, J.C.; Cottrell, S.J.; Feeder, N.; Galek, P.T.A.; Groom, C.R.; Pidcock, E. Evaluation of molecular crystal structures using Full Interaction Maps. Cryst. Eng. Comm. 2013, 15, 65–72. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta. Crystallogr. B. Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Cambridge Structural Database System, Version 5.41; Cambridge Crystallographic Data Centre: Cambridge, UK, 2020.
- Canossa, S.; Predieri, G.; Graiff, C. Hydrogen bonds and π-π Interactions in two new crystalline phases of methylene blue. Acta Crystallogr. Sect. E Crystallogr. Commun. 2018, 74, 587–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rager, T.; Geoffroy, A.; Hilfiker, R.; Storey, J.M.D. The crystalline state of methylene blue: A zoo of hydrates. Phys. Chem. Chem. Phys. 2012, 14, 8074–8082. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.D.; Arora, K.K.; Bass, H.; Kavuru, P.; Ong, T.T.; Pujari, T.; Wojtas, L.; Zaworotko, M.J. Structure-stability relationships in cocrystal hydrates: Does the promiscuity of water make crystalline hydrates the nemesis of crystal engineering? Cryst. Growth Des. 2010, 10, 2152–2167. [Google Scholar] [CrossRef]
- Lausi, A.; Polentarutti, M.; Onesti, S.; Plaisier, J.R.; Busetto, E.; Bais, G.; Barba, L.; Cassetta, A.; Campi, G.; Lamba, D.; et al. Status of the crystallography beamlines at Elettra. Eur. Phys. J. Plus 2015, 130, 1–8. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System, Version 1.171.40.67a; Rigaku Oxford Diffraction: Oxford, UK, 2017.
- Bruker AXS. APEX3 Software, Version 2016.1-0; Bruker AXS: Madison, WI, USA, 2016.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer, Version 17.05; University of Western Australia: Perth, Australia, 2012.
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. Cryst. Eng. Comm. 2009, 11, 19–32. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr. Sect. B Struct. Sci 2004, 60, 627–668. [Google Scholar] [CrossRef]
- Durão, J.; Gales, L. Permeation of Light Gases through Hexagonal Ice. Materials 2012, 5, 1593–1601. [Google Scholar] [CrossRef]
- Corpinot, M.K.; Bučar, D.K. A Practical Guide to the Design of Molecular Crystals. Cryst. Growth Des. 2019, 19, 1426–1453. [Google Scholar] [CrossRef] [Green Version]









| Phase | Void Volume (Å3) | Void Fraction (%) | Atomic Packing Factor |
|---|---|---|---|
| MB2[Ni(CN)4]·2H2O | 980.74 | 26.9 | 0.731 |
| MB2[Fe(CN)5NO] | 445.26 | 24.8 | 0.752 |
| MB3[Fe(CN)6]·H2O | 585.17 | 23.5 | 0.765 |
| MB3[Fe(CN)6]·12.36H2O | 378.07 | 24.6 | 0.754 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canossa, S.; Graiff, C.; Crocco, D.; Predieri, G. Water Structures and Packing Efficiency in Methylene Blue Cyanometallate Salts. Crystals 2020, 10, 558. https://doi.org/10.3390/cryst10070558
Canossa S, Graiff C, Crocco D, Predieri G. Water Structures and Packing Efficiency in Methylene Blue Cyanometallate Salts. Crystals. 2020; 10(7):558. https://doi.org/10.3390/cryst10070558
Chicago/Turabian StyleCanossa, Stefano, Claudia Graiff, Domenico Crocco, and Giovanni Predieri. 2020. "Water Structures and Packing Efficiency in Methylene Blue Cyanometallate Salts" Crystals 10, no. 7: 558. https://doi.org/10.3390/cryst10070558