Prostaglandin E1-Mediated Collateral Recruitment Is Delayed in a Neonatal Rat Stroke Model
Abstract
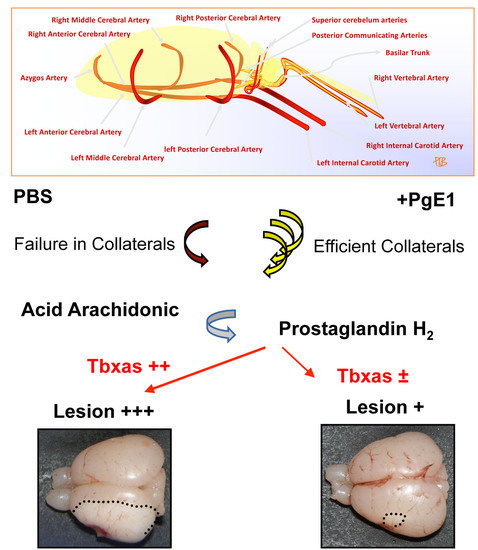
:1. Introduction
2. Results
2.1. Neonatal Ischemia Induces Thromboxane a Synthase-1 Gene Expression
2.2. PgE1 Induces Delayed Blood-Flow Redistribution in the Ipsilateral Side
2.3. PgE1 Reduces Thromboxane a Synthase-1 Expression and Lesion Size
3. Discussion
4. Materials and Methods
4.1. Neonatal Ischemia-Reperfusion
4.2. Drug Treatment
4.3. Sample Size Calculation
4.4. Quantitative Polymerase Chain Reaction Analysis
4.5. Ultrasound Imaging
4.6. Measurement of Infarct Volume
4.7. Immunohistochemistry
4.8. Statistical Analysis
Supplementary Materials
Author contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wagenaar, N.; Martinez-Biarge, M.; van der Aa, N.E.; van Haastert, I.C.; Groenendaal, F.; Benders, M.J.N.L.; Cowan, F.M.; de Vries, L.S. Neurodevelopment after perinatal arterial ischemic stroke. Pediatrics 2018, 142, e20174164. [Google Scholar] [CrossRef] [PubMed]
- Van Bel, F.; Dorrepaal, C.A.; Benders, M.J.; Zeeuwe, P.E.; van de Bor, M.; Berger, H.M. Changes in cerebral hemodynamics and oxygenation in the first 24 h after birth asphyxia. Pediatrics 1993, 92, 365–372. [Google Scholar] [PubMed]
- Charriaut-Marlangue, C.; Bonnin, P.; Gharib, A.; Leger, P.L.; Villapol, S.; Pocard, M.; Gressens, P.; Renolleau, S.; Baud, O. Inhaled nitric oxide reduces brain damage by collateral recruitment in a neonatal stroke model. Stroke 2012, 43, 3078–3084. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; Macvicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renolleau, S.; Aggoun-Zouaoui, D.; Ben-Ari, Y.; Charriaut-Marlangue, C. A model of transient unilateral focal ischemia with reperfusion in the P7 neonatal rat: Morphological changes indicative of apoptosis. Stroke 1998, 29, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, P.; Leger, P.L.; Deroide, N.; Fau, S.; Baud, O.; Pocard, M.; Charriaut-Marlangue, C.; Renolleau, S. Impact of intracranial blood-flow redistribution on stroke size during ischemia-reperfusion in 7-day-old rats. J. Neurosci. Meth. 2011, 198, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Leger, P.L.; Pansiot, J.; Besson, V.C.; Palmier, B.; Renolleau, S.; Baud, O.; Cauli, B.; Charriaut-Marlangue, C. Cyclooxygenase-2-derived prostaglandins mediate cerebral microcirculation in a juvenile ischemic rat model. Stroke 2016, 47, 3048–3052. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, P.; Pansiot, J.; Paven, E.; Eloi, M.; Renolleau, S.; Baud, O.; Leger, P.L.; Charriaut-Marlangue, C. Controlled arterial blood flow after ischemia induces better outcomes in the juvenile rat brain. J. Cerebr. Blood Flow Metabol. 2017, 37, 3091–3096. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Iida, H.; Iida, M.; Uchida, M.; Dohi, S. The comparative effects of prostaglandin E1 and nicardipine on cerebralmicrocirculation in rabbits. Anesth. Analg. 2003, 96, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Beretta, S.; Versace, A.; Carone, D.; Riva, M.; Dell’Era, V.; Cuccione, E.; Cai, R.; Monza, L.; Pirovano, S.; Padovano, G.; et al. Cerebral collateral therapeutics in acute ischemic stroke: A randomized preclinical trial of four modulation strategies. J. Cerebr. Blood Flow Metabol. 2017, 37, 3344–3354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollace, V.; Muscoli, C.; Masini, E.; Cuzzocrea, S.; Salvemini, D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol. Rev. 2005, 57, 217–252. [Google Scholar] [CrossRef] [PubMed]
- Leger, P.L.; Bonnin, P.; Lacombe, P.; Couture-Lepetit, E.; Fau, S.; Renolleau, S.; Baud, O.; Charriaut-Marlangue, C. Dynamic spatio-temporal imaging of early reflow in a neonatal stroke model. J. Cerebr. Blood Flow Metabol. 2013, 33, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Leger, P.L.; Bonnin, P.; Moretti, R.; Tanaka, S.; Duranteau, J.; Renolleau, S.; Baud, O.; Charriaut-Marlangue, C. Early recruitment of cerebral microcirculation by neuronal nitric oxide synthase in a juvenile ischemic rat model. Cerebrovasc. Dis. 2016, 41, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, B.A.; Papadakis, M.; Chen, R.L.; Buchan, A.M. Cerebral blood flow alteration in neuroprotection following cerebral ischemia. J. Physiol. 2011, 589, 4105–4114. [Google Scholar] [CrossRef] [PubMed]
- Charriaut-Marlangue, C.; Baud, O. A model of perinatal stroke: 20 years already and what lessons? Front. Neurol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Schror, K.; Hohlfeld, T. Mechanisms of anti-ischemic action of prostaglandin E1 in peripheral arterial occlusive disease. Vasa 2004, 33, 119–124. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnin, P.; Pansiot, J.; Baud, O.; Charriaut-Marlangue, C. Prostaglandin E1-Mediated Collateral Recruitment Is Delayed in a Neonatal Rat Stroke Model. Int. J. Mol. Sci. 2018, 19, 2995. https://doi.org/10.3390/ijms19102995
Bonnin P, Pansiot J, Baud O, Charriaut-Marlangue C. Prostaglandin E1-Mediated Collateral Recruitment Is Delayed in a Neonatal Rat Stroke Model. International Journal of Molecular Sciences. 2018; 19(10):2995. https://doi.org/10.3390/ijms19102995
Chicago/Turabian StyleBonnin, Philippe, Julien Pansiot, Olivier Baud, and Christiane Charriaut-Marlangue. 2018. "Prostaglandin E1-Mediated Collateral Recruitment Is Delayed in a Neonatal Rat Stroke Model" International Journal of Molecular Sciences 19, no. 10: 2995. https://doi.org/10.3390/ijms19102995